Article last updated: 14 April 2018 (dangers of the IQOS)
The idea behind heated tobacco in the IQOS

The IQOS is an electronic device that heats tobacco without burning it.
The idea behind heated tobacco is based on the fact that it’s the burning of tobacco that produces the thousands of highly toxic compounds in cigarette smoke. These substances include around sixty known carcinogens, making smoking the leading cause of premature death worldwide.
To avoid burning the tobacco, scientists at Philip Morris International developed a new tobacco mix that releases a nicotine aerosol at a relatively low temperature when it’s used in an electronic device. The key word here is “relatively”, because the IQOS still heats this new tobacco mix, but only to 350°C. That means it doesn’t burn, so in theory it won’t produce the multitude of substances found in tobacco smoke, like carbon monoxide, tar, nitrosamines, cadmium, polycyclic hydrocarbons and even mercury.
We don’t know the exact composition of the tobacco in the sticks, i.e the small cigarettes known as “heets” or “teeps” depending on which country they’re sold in, and which were sometimes branded as Marlboro when they were first sold.
As far as we know, the mixture appears to contain at least one humidifying agent (like glycerine or propylene). Beyond that, as a curious consumer you might imagine any number of possible ingredients, but the recipe is a well-guarded industrial secret. The mystery surrounding the manufacture of tobacco cigarettes – specifically any additives they may contain – has unfortunately cast doubt on the properties of the tobacco used in the IQOS.
Two independent studies detect harmful compounds
Philip Morris regularly publishes data from its own studies. To date, only one independent study about the IQOS has been published – by the journal JAMA Internal Medicine, on 22 May 2017. Researchers at the University of Lausanne in Switzerland have also been questioning whether the IQOS really is smoke-free. After analysing the chemicals released by the device, their findings contest the marketing ad so frequently cited by the media. According to the study conducted by Prof. Reto Auer, “The smoke from the IQOS contains compounds caused by pyrolysis and thermochemical degradation, which are the same harmful compounds as in conventional tobacco cigarette smoke.”
In its response published on its website on 30 May 2017, Philip Morris acknowledges that pyrolysis takes place. “We have never claimed that the IQOS does not use pyrolysis, which is well known to increase with rising temperature, and which produces most of the harmful or potentially harmful compounds (HPHC) found in the IQOS aerosol. However, there is no combustion in the IQOS,” explain Serge Maeder and Manuel Peitsch, scientists working for the cigarette manufacturer.
The Lausanne researchers, who conducted this study without funding after the Swiss Tobacco Prevention Fund (CPT) refused any financial assistance, assert that because pyrolysis occurs in the IQOS – as it does in conventional cigarettes – we should consider the compounds it releases to be smoke. “Volatile organic compounds (VOC), polycyclic aromatic hydrocarbons (PAH) and carbon monoxide (CO) were present in the smoke from the IQOS,” summarises the article in the journal JAMA Internal Medicine.
The Lausanne researchers also compared the relative amounts of toxic emissions from the IQOS with a Lucky Strike Blue Light cigarette. In IQOS smoke, they found the equivalent of 82% of the acrolein released by the Lucky Strike, 74% of the formaldehyde, 50% of the benzaldehyde and nearly three times as much acenaphthylene (a tar not always measured in studies). These are significantly higher ratios than those stated in the tobacco company’s own studies.
Scientists at Philip Morris have cast doubt on these results, which they consider “very surprising” on methodological grounds. They have accused the Lausanne researchers of failing to follow standard study procedures for the subject, which usually use standard smoking machines and cigarettes (specifically the 3R4F). In their view, the measurements published by the independent researchers show variations compared to previous studies, thereby indicating that their “method of analysis may be flawed”. In a letter received on 8 June 2017 by Jean-Daniel Tissot, Dean of the Faculty of Biology and Medicine at the University of Lausanne, Philip Morris asked for the study to be withdrawn.
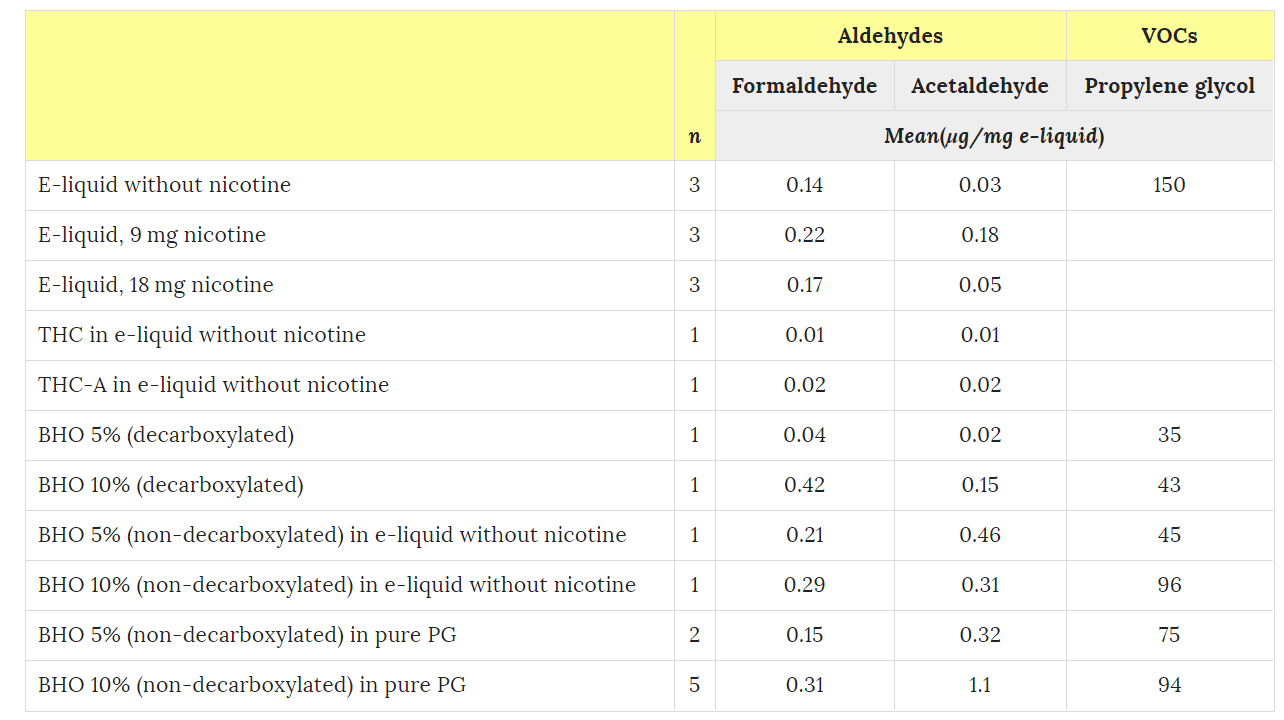
Scientific Reports 6, Article number: 25599 (2016)
doi:10.1038/srep25599
It’s worth noting that the testing machine used by the Lausanne researchers had also been used for a study on vaping, specifically with cannabis, that was published in Nature in May 2016. During that study, several vape aerosols were analysed (without and with different levels of nicotine and, moreover, with and without cannabis extract). These did not indicate any presence of polycyclic aromatic hydrocarbons (PAH), and showed insignificant levels of volatile organic compounds (VOC), with the exception of propylene glycol – no surprise there.
The scientific debate is set to continue and has become political too, given the commercial aspects, as reported by the Vapolitique blog.
In November 2017, Jean-François Etter from the Global Institute of Public Health at the University of Geneva reported that the authors of the independent Lausanne study had responded to the manufacturer.
“The authors of the Lausanne study, who have claimed that the heated tobacco product IQOS produces smoke, have responded to manufacturer Philip Morris International (PMI), which disputes this claim. This response is accompanied by an editorial from the journal JAMA Internal Medicine criticising the pressure PMI has put on these researchers and their University authorities, and the intimidation PMI has used against the researchers.”
https://jamanetwork.com/journals/jamainternalmedicine/article-abstract/2661701 and https://jamanetwork.com/journals/jamainternalmedicine/article-abstract/2660130
The second study which detected potentially harmful emissions when using the IQOS was carried out “by a laboratory commissioned by the Blue Cross of Bern, Soleure and Friburg”, a Swiss TV news show reported.
It claimed that “dangerous toxins” called isocyanates are released when the device’s polymer filters are heated to temperatures above 100°C. These are found specifically in certain solvents, coatings, paints and other industrial foams.
In their study, the scientists heated the filters to temperatures of 100, 160 and 200 degrees. The manufacturer says that when using its product, tobacco is heated to over 300°.
The question is whether smokers inhale these toxins.
The tobacco company’s spokesperson was well aware of this when asked about it. However, he was keen to point out that IQOS users do not inhale these toxins under any circumstances.
Either way, this study raises new questions about just how much risk PMI’s product reduces compared to smoking tobacco.
Competitor detects volatile organic compounds
To date, only one of PMI’s competitors, Imperial Tobacco, has been able to critically – and a priori, scientifically – review the IQOS. It stated that it detected traces of certain volatile organic compounds (VOC) in secondary smoke. Imperial’s development strategy hasn’t included heated tobacco technology. Instead it uses a hybrid process for e-cigarettes, so it would be wise to take its conclusions with a pinch of salt, especially as their findings have not yet been published in any scientific journal.
According to one American government agency, PMI recently submitted a request for certification for the IQOS on the basis that it poses less of a risk than tobacco. This scientific and legal dossier apparently contains 3 million pages, the equivalent of 15 tons of paper, which when stacked would be 1.5 km high. The product is already marketed in around twenty countries and has been on sale in French tobacconists since May 2017.
From a sales perspective, Philip Morris is clearly looking to reposition itself in the risk reduction market. Is this a paradox? Not entirely.
No ethics in business
Should we believe that this industry giant has suddenly been overcome with such guilt that it’s trying to make up for its past mistakes?
These days, it’s consumer choice that dominates our economy, especially now that everyone is online and shares information. If consumers want to eat more healthily, the market will adapt naturally. Attracted by a new source of profit, small, flexible and fast-moving businesses will quickly move into this new gap in the market, with larger companies following later as economic indicators confirm the potential profit to be made and the stability of the new market. The explosion of the organic market, for example, shows how a change in demand can cause fast, far-reaching transformations in a well-established economic sector. Tobacco is bound to follow the same rule.
Waiting for the right moment
“Heets” tobacco sticks were once branded for sale as Marlboro.
As you may imagine, Philip Morris didn’t come up with the IQOS yesterday. Designed in its research centre in Neuchâtel, Switzerland, the IQOS is the realisation of a technical concept first thought up by the tobacco industry in the 1980s.
Manufacturers selling heated tobacco products include Reynolds American (RAI) with its Eclipse, REVO and the Core, Philip Morris International with its HeatStick (also called “HEETs” and now “TEEPs”) and its IQOS (“I Quit Ordinary Smoking ”), British American Tobacco (BAT) with the Glo and Glo iFuse, and Japan Tobacco (JTI) with its Ploom, and PAX concept.
Since the late 1980s, these products have all failed. One of these was Philip Morris’s 1998 Accord, which even then looked a lot like the IQOS.
New players are trying to break into this market, including Chinese wholesaler Heaven Gifts, which specialises in vaping products. Its catalogue features the “iBuddy” heating kit, but they don’t actually sell tobacco sticks.
Hon Lik was the first to introduce the mass-produced vape (e-cigarette) in 2003. It paved the way for these new products to develop. The massive popularity of e-cigarettes among consumers really raised awareness of consuming non-tobacco products. The creation of regulatory frameworks gave the green light to the marketing departments at big-name tobacco companies to step up and jump feet first into the risk reduction market.
Since then, the media has caused confusion between heated tobacco cigarettes and e-cigarettes, describing both as “electronic cigarettes”.
This was good news for tobacco company Philip Morris. Consumers were already aware of the benefits of e-cigarettes compared to smoked tobacco, so were certainly more inclined to try the new product from the makers of Marlboro on sale in tobacconists’ shops. But does the IQOS come anywhere close to an e-cigarette in reality?
The IQOS is not an e-cigarette

Some IQOS Heets cigarette packets read, “The pleasure of tobacco without the ash or the strong smell.”
The term “e-cigarette” has always posed a challenge semantically, since the word “cigarette” is so strongly associated with tobacco, in contrast to the anti-tobacco stance of the e-cigarette. However, the name remains part of our vocabulary. One thing’s for sure: the IQOS does not use e-liquid and doesn’t come in lots of different versions.
Another key detail is that the IQOS is not – even indirectly – marketed as a smoking cessation tool. Unlike the e-cigarette market, where vendors regularly (indirectly) tell smokers about how effective they are for smoking cessation, the IQOS is designed to make you smoke differently. The acronym IQOS stands for “I Quit Ordinary Smoking”, so there’s no doubt about the intention behind the marketing approach for this new tobacco product.
Any tobacco company’s business is based on the highly addictive nature of its products. If a smoker buys an IQOS instead of their usual cigarettes, from a marketing perspective they want that consumer to keep buying heated tobacco sticks, not to stop after a few months. This commercial interest in keeping customers captive may hold a clue to the composition of the product.
Is the IQOS any less dangerous than traditional cigarettes?

In December 2016, Jan Heide, a researcher from the University of Rostock in Germany, addressed the Ecig Symposium in La Rochelle, France. He presented some of his findings from his work on smokeless tobacco products.
If there’s no burning involved, it seems logical that IQOS would reduce risk compared to smoked tobacco, because the vast majority of the dangerous compounds in tobacco smoke come from burning it.
To date, the only data available about risk reduction is the data presented by Philip Morris International itself. And as everyone knows, the tumultuous history of the tobacco industry means that most of the scientific community is rather wary of its claims. On the other hand, no one can deny the colossal scale of PMI’s scientific undertakings.
To date, only the University of Rostock in Germany (photo above) have studied comparative analyses between conventional tobacco and heated tobacco. Some of their conclusions were presented in brief at the first Ecig Symposium in La Rochelle in December 2016, but have not yet been published in a scientific journal. Initial findings do seem to indicate compound levels below those found in tobacco smoke, but without “creating or reducing certain dangerous or very dangerous compounds”.
The Greek cardiologist Konstantinos Farsalinos has also worked on the subject. His key conclusions were presented at Vapexpo in Lyon in March 2017. They are as follows:
- Heated tobacco products are an old concept that was unsuccessful in the past.
- New developments and new products are already on the market, or soon will be.
- Heated tobacco is very similar to tobacco cigarettes in terms of smoking behaviour.
- There is no second-hand smoke but there is an unpleasant odour (very similar to tobacco smoke).
- There’s a weak hit in the throat.
- There are fewer toxins in heated tobacco smoke than in tobacco smoked via burning, but far more than in the latest generation of e-cigarettes.
- Vapers won’t like heated tobacco cigarettes, but they may be a viable alternative for smokers who sometimes vape or smokers who don’t like e-cigarettes.
One Swiss organisation is concerned about issues revealed by a study it commissioned. These include IQOS filter elements melting during use, and the presence of polylactic acid (PLA), a bio-based, biodegradable polymer. Philip Morris maintains that “all of the evidence available today clearly shows that the IQOS is a better alternative for smokers who would otherwise continue to smoke cigarettes.”
In the absence of any independent studies, however, it is impossible to formally claim that the IQOS reduces the risks of smoked tobacco. Nevertheless, in a report published in February 2018 on heated tobacco, and which reviews the IQOS in depth, the British public health agency (PHE) considers these products to be safer than smoked cigarettes but not as safe as vaping. The PHE remains very cautious, and will do until there has been more independent research.
Is the IQOS a safer alternative to e-cigarettes?
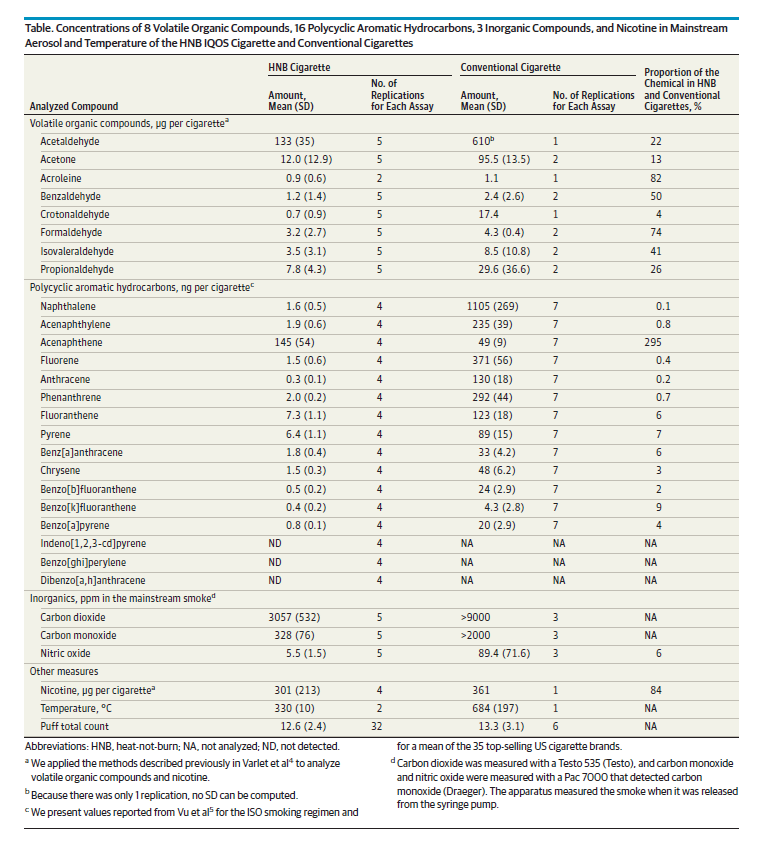
The report by Public Health England rates the toxicity of heated tobacco in general, and of the IQOS in particular, as somewhere between traditional cigarettes and e-cigarettes. Given the lack of independent research, it has shied away from making a more conclusive assessment. In March 2017, during a presentation at Vapexpo in Lyon, Farsalinos said, “No, the IQOS is not a safer alternative to a modern e-cigarette which has fewer toxins in its vapour.”
Here are three posters presented at Vapexpo in Lyon in March 2017:
The important thing to remember at this stage in the scientific research is that the ingredients in e-liquids for e-cigarettes are clearly identified and well known. It’s a different story with the heated tobacco in the IQOS because the manufacturing methods for this new tobacco are completely confidential.
The second point to consider is the number of studies on e-cigarettes published to date in scientific journals. This has a bearing on the admissibility of a publication. Of course, not all published studies are equal. But the more there are, the easier it will be to systematically review and analyse the scientific literature in depth, allowing scientists to develop a knowledge base. Reports by PHE, RCP and more recently by Truth Initiative (covering almost 700 studies) are excellent benchmarks.
Toxicology studies on e-cigarettes overwhelmingly show specific, significant risk reduction compared to smoked tobacco. Researchers remain concerned about a small number of compounds which still provoke debate, primarily aldehydes. Another major worry among researchers is the dual use of vaping/tobacco. In some countries, this applies to large numbers of vapers. Finally, there is serious debate about long-term effects.
Is the IQOS really a smokeless cigarette?
Auer R, Concha-Lozano N, Jacot-Sadowski I, Cornuz J, Berthet A. Heat-Not-Burn Tobacco Cigarettes: Smoke by Any Other Name. JAMA Intern Med. Published online May 22, 2017. doi:10.1001/jamainternmed.2017.1419
Conclusion
In our opinion, Philip Morris International’s IQOS is not an e-cigarette as we know it but a tobacco product. While the IQOS may reduce risk compared to smoked tobacco, the only data available has been published by Philip Morris International itself. The lack of independent data certainly calls for caution, and means that the IQOS cannot be scientifically compared to a conventional e-cigarette.
On the other hand, we now know a lot more about e-cigarettes, and the reduction in risk they offer has been recognised by a growing number of public health organizations.
With its ten-digit turnover, PMI seems rather far-removed from the social movement, partially started by vapers, which has allowed great strides in risk reduction from smoking in recent years.
PMI seeking “modified risk” status
On 5 December 2016, Philip Morris International announced it was filing a “Modified Risk Tobacco Product” (MRTP) application with the FDA. That would give the IQOS reduced risk certification.
After examining the many documents submitted by PMI, on 30 April 2019 the FDA officially authorised the tobacco company to market its product in the US. However, its decision on “modified risk” status is still pending.