Juul Labs is back with a new closed-system pod device that it calls a solution to address a variety of issues, like youth uptake of vaping

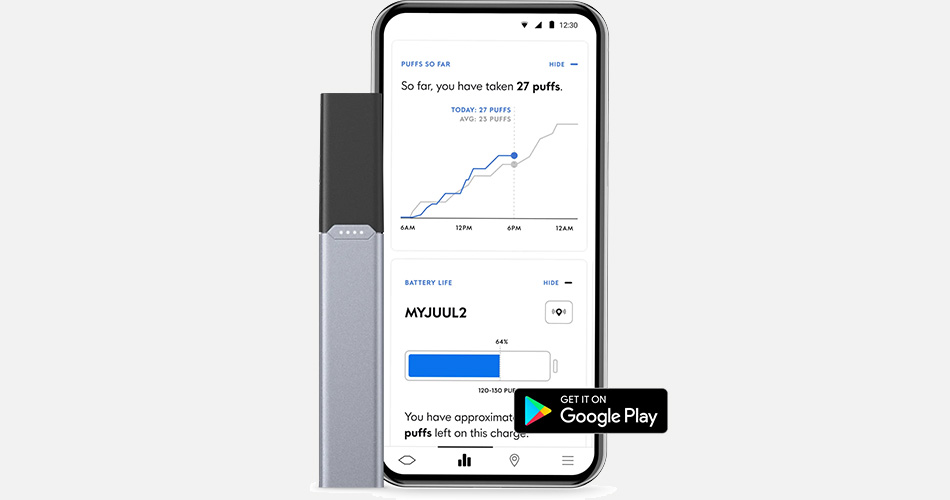
Juul Labs is hardly a saint in the e-cigarette industry. However, the company wants to change its image with a brand new iteration of its iconic USB-style closed system. In an announcement recently published by the company, Juul has filed a premarket tobacco application for the Juul2 system—a new e-cigarette with age verification tech built in.
“Our company DNA is product innovation,” said Kirk Phelps, Juul’s chief product officer, in a press statement, claiming they have “designed a technological solution for two public-health problems.” These problems, according to Phelps, are improving the ability of adult smokers to switch from combustible cigarettes to risk-reduced e-vapor products and to prevent and combat underage e-cigarette use in the U.S. market. Juul2 was tested and initially launched in the United Kingdom. Features of the system include a method for unique pod identification to combat illicit and counterfeit pods. The only flavor for Juul2 is a tobacco-characterized pod with liquid that tastes like “Virginia tobacco.” But, the selling point that Juul hopes will impress regulators at the Center for Tobacco Products and the Food and Drug Administration is the incorporation of new age verification technologies.
Meet Juul2

A technical description released by Juul points out that the Juul2 system will be fully integrated with a mobile and web-based portfolio of applications that allows users to verify their ages, lock devices, and report usage insights on consumers in real-time. Honestly, I find the real-time data reporting a bit sketchy but Juul has taken a step that I can support. There is no denying that I, with millions of other consumers in the vaping industry, have negative opinions on Juul. Remember, I called Juul Labs the “Walmart of vaping.” But, after reviewing everything that I could locate on the Juul2 system and its success in the United Kingdom provides a promising future for the company and users.
Despite record declines in cases of minors reporting e-cigarette use, one of the go-to talking points for the tobacco control and anti-vaping movements is to point out that vaping devices—particularly Juul devices—were marketed to children. The evidence used by these organizations to argue in favor of this claim is built on outdated data and marketing trends that Juul incorporated at the launch of its product years ago. After a moral panic about vaping that was stoked by former FDA commissioner Scott Gottlieb and other Trump public health officials, the end result was Juul settling literally thousands of lawsuits filed by school districts, personal injury lawyers, and governments for marketing and, by consequence, “addicting a new generation.” Vaping Post has reported extensively over the years that many of these claims are extremely dubious and are a result of the extremely litigious culture of the United States. Due to this controversy focused on Juul and its quick dominance of the vaping market, the company found enemies on all sides.
A new chapter?
Juul2, however, could make up for the company’s failures over the years. Integrating the age verification protocols, even after a sale is made, is not perfect but it is a strategy that allows adult users to self-regulate their personal use and use around minors. This new product feature will require an extensive educational campaign to inform consumers of the features. While this concept isn’t novel, the Juul2 age verification functionality will be marketed to great lengths by the manufacturer. And, consumer marketing basics require a clear roadmap to ensure compliance with FDA advertising regulations, product safety, and public health regulations. With the filing of a new PMTA application, Juul will start the process of getting its new system approved just as if it were any other deemed tobacco product. As we’ve seen, PMTA is a costly and time-intensive process for manufacturers.
This was one of the criticisms of the PMTA rule and its potential to harm small- and medium-sized manufacturers across the country. Juul is still a huge company, though. So, the cost restrictions will have very little impact on the potential for product approval. But, at the heart of the issue is whether Juul learned its lessons from its biggest blunder to date. As a reminder, the FDA issued a marketing denial order blocking the sale of all Juul products in the United States market. A stay on the order was granted in response to a temporary administrative stay that was issued by the U.S. Court of Appeals for the D.C. Circuit and an agency-initiated stay to review “scientific issues” specific to the Juul PMTA.
Hanging by a thread
With these stays in place, Juul products are still available for purchase by consumers who meet the minimum legal sales age of 21 years or older. In the regulatory and scientific tit-for-tat for the Juul2 application, representatives working on behalf of the company need to go beyond their renewed commitment to regulatory compliance, and then some, to show the FDA that Juul is an ethical, compliant, and transparent company. Joe Murillo, the chief regulatory officer for Juul Labs, said that this is the intention of the Juul2 application.
“Our next-generation vapor platform PMTA is built on new technology that advances public-health objectives and compelling science that demonstrates a clear public-health benefit, as required to secure a marketing authorization,” said Mr. Murillo, in the same press statement I referenced already. “We look forward to engaging with FDA throughout the review process while we pursue this important harm-reduction opportunity.” Based on this information, Juul has an opportunity as I said. They can’t mess it up. If they do, regardless of it being the regulatory hurdles or the company itself, Juul Labs will be done in the U.S. market. And, this is a sentiment that hangs over many of my friends who have worked and still work for Juul, despite the tumultuous track record the company has had since 2018. I also implore the Food and Drug Administration to approve Juul2 for the U.S. market. Approving a device like Juul2 has more net benefits for improving public health.











